
In this second article of our five-part series on culinary herb research at Michigan State University (MSU), we will highlight the results of pushing the lighting and carbon dioxide envelope during the production of dill, parsley, and sage seedlings indoors without sunlight. To see part one, click here.
Increasing light intensity up to a certain species-specific point increases the yield (ex. fresh mass) and quality of many crops including culinary herbs. However, what if adding supplemental lighting in the greenhouse or increasing the intensity of sole-source lighting in indoor (vertical farms, warehouses and containers) production for the entire crop production duration is not economically feasible? Dynamic environmental control, or adjusting the growing environment based on plant age or production stage, could be more efficient in enhancing crop yield and quality and reducing production costs.
With more expensive capital and operating costs for indoor specialty crop production, short duration and high-density crops are potentially higher-profit options. With this higher cost of production comes increased environmental control capabilities, including the ability to manipulate sole-source light intensity and quality, temperature, humidity, and carbon dioxide (CO2) concentration. The question is, how do we leverage this control effectively?
In this study, we focus on indoor herb young plant production, from seed sowing to transplant. Our hypothesis was that increasing production inputs such as light and CO2 during the young plant stage could give those plants a head start for the finishing stages. Additionally, during young plant production, plant density is much higher than during finished production. For example, a 200-cell tray of culinary herb seedlings can be produced in two weeks in the same area that three to 20 plants can be grown during finishing stages and harvested in three weeks. If the higher operating and capital cost of indoor production can be spread across a larger number of plants with a shorter production duration, the cost per plant is much less.
In our previous indoor seedling production research focused on basil, we determined that seedling fresh mass was 284% greater when grown under 600 µmol·m?2·s?1 (daily light integral [DLI] of 35 mol·m?2·d?1) compared to 100 µmol·m?2·s?1 (DLI of 6 mol·m?2·d?1). However, increasing CO2 concentration did not increase fresh mass of basil seedlings. More importantly, after being transplanted into a common greenhouse environment, an 80% increase in fresh mass persisted through harvest. This was a large increase in harvestable yield and we wanted to determine if other commonly grown culinary herbs would have a similar response.


Seedling production
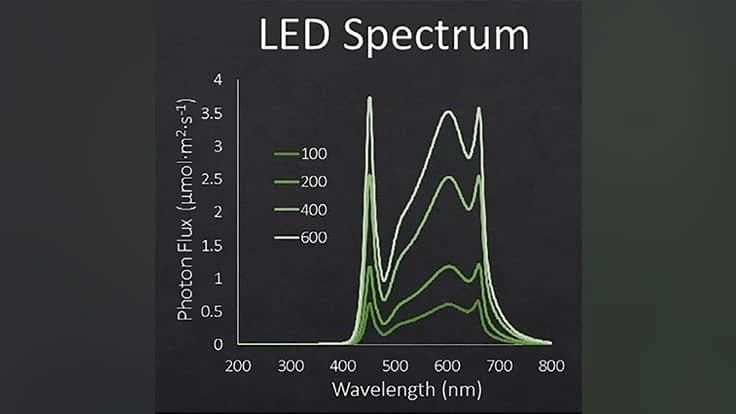
We selected three popular culinary herbs, dill ‘Bouquet’, parsley ‘Giant of Italy’, and Sage ‘Extrakta.’ Seeds were sown in 200-cell rockwool cubes and placed in walk-in growth rooms with CO2 concentrations of 400, 800 or 1200 ppm. Each chamber was equipped with light-emitting diodes (LEDs) that provided four different light intensities of 100, 200, 400, and 600 µmol·m–2·s–1 (Fig. 1). The LEDs were on 16 hours each day to create DLIs of 6, 12, 24 and 35 mol·m–2·d–1. After three (dill), four (sage), or five (parsley) weeks after sowing, data was collected.
Increasing indoor light intensity from 100 to 600 µmol·m–2·s–1 had different effects depending on the genera and CO2 concentration. The fresh mass of parsley seedlings was 12% greater when grown under 100 µmol·m–2·s–1 compared to 600 µmol·m–2·s–1 (Fig. 2). The opposite trend occurred for sage seedlings where those grown under 400 µmol·m–2·s–1, had 17% greater fresh mass than those grown under 100 µmol·m–2·s–1 (Fig. 3). The influence of light intensity on dill fresh mass depended upon the CO2 concentration provided (Fig. 4). When CO2 concentration was elevated to 1200 ppm, plants grown under 600 µmol·m?2·s?1 had the greatest fresh mass. When CO2 concentration was not elevated above ambient levels (400 ppm), plants grown under 400 µmol·m–2·s–1 had the greatest mass.
Besides fresh mass, another important factor to consider is seedling compactness. Seedlings grown under 100 µmol·m?2·s?1 tended to be floppy, elongated, and tangle together. Increasing light intensity resulted in more compact and sturdy seedlings that would potentially utilize less labor or be easier to automate transplanting.
Crops responded differently to elevated CO2 as well. Increasing CO2 concentrations did not influence the fresh mass of sage, but did increase the fresh mass of parsley. The greater CO2 input increased fresh mass of dill only when the light intensity was high (600 µmol·m–2·s–1).

Finished production
You may be thinking: Great, you have increased fresh mass at transplant, but that is not the product I am selling! That was our thought as well, so we transplanted these seedlings into deep flow raft hydroponic systems and grew the plants in a common greenhouse environment with an average daily temperature of 73 °F and DLI of 14 mol·m–2·d–1 for four (dill and sage) or five (parsley) weeks.
We found that elevating the CO2 concentration during the seedling stage did not influence any of the crops at harvest. However, increasing light intensity from 100 to 400 µmol·m?2·s?1 increased dill and sage yield by 47% and 69%, respectively (Fig. 5). Additionally, when dill seedlings were grown under higher light intensities, the stems were thicker and plants were less floppy at harvest (Fig. 6).

Efficiency implications
By sowing seeds in a 200-cell tray (200 in2 per flat, 1 in2 per cell), the planting density is 144 plants per ft2. Seedlings were transplanted 8-in apart with a planting density of 2.25 plants per f2. Therefore, the planting density was 64 times greater during seedling production than finished (harvest) production. Additionally, in this study, the duration of dill seedling production was 3/4 that of finishing (three weeks compared to four weeks). Taking both the increased planting density and shorter production duration into account, the increase in sole-source lighting cost per plant could be discounted by 64 (sage) to 85 (dill) times during seedling versus finished production.
Therefore, in this case, the cost per plant of increasing the light intensity from 100 to 400 µmol·m?2·s?1 during propagation was ~4 to 5% that of the cost during finished production. Since the increase in yield at the finishing stage was 47% (dill) and 69% (sage) greater when seedlings were grown under 400 µmol·m–2·s–1 compared to 100 µmol·m–2·s–1 (24 compared 6 mol·m?2·d?1), increasing light intensity during seedling production increases subsequent yield while reducing input costs.

The bottom line
Elevating CO2 concentration during young plant production did not have a long-term effect on plant growth or harvestable yield of the culinary herbs we have investigated. However, increasing light intensity during dill and sage seedling production, but not parsley, is a more effective strategy to increase subsequent yield.

Explore the December 2020 Issue
Check out more from this issue and find your next story to read.
Latest from Produce Grower
- University of Evansville launches 'We Grow Aces!' to tackle food insecurity with anu, eko Solutions
- LettUs Grow, KG Systems partner on Advanced Aeroponics technology
- Find out what's in FMI's Power of Produce 2025 report
- The Growth Industry Episode 3: Across the Pond with Neville Stein
- The Growth Industry Episode 2: Emily Showalter on how Willoway Nurseries transformed its business
- 80 Acres Farms expands to Georgia, Texas and Colorado
- How BrightFarms quadrupled capacity in six months
- Oasis Grower Solutions releases two foam AeroSubstrates for hydroponic growers






